Data-Driven Trends In Predetermined Change Control Plans
- Yu Zhao
- Jul 12, 2024
- 9 min read
Updated: Jul 28, 2024
The FDA introduced the concept of the Predetermined Change Control Plan (PCCP) to manage the dynamic nature of postmarket changes to medical devices and issued a draft guidance in April 2023 regarding PCCP for AI/ML-enabled software functions. An authorized PCCP allows the FDA to pre-clear or pre-approve predefined specific changes to previously cleared or approved devices without the need for a new premarket submission for each change. This is contingent upon the manufacturer following predetermined development, verification, validation, and implementation protocols.
Bridging Consulting recently conducted a data-driven study on PCCPs. Yu Zhao, Founder and President of Bridging Consulting, shares the key findings in a new guest column article on Med Device Online: Data-Driven Trends In Predetermined Change Control Plans.

Image by Gerd Altmann from Pixabay
Data-Driven Trends In Predetermined Change Control Plans
In the ever-evolving landscape of medical device regulations, the Predetermined Change Control Plan (PCCP) represents a significant advancement in how the U.S. FDA manages postmarket changes to medical devices. This article will provide an update on the status of the PCCP, examine regulatory developments, analyze authorized PCCPs, and explore emerging trends and future directions.
The concept of the PCCP was introduced to address the dynamic nature of medical devices, particularly those utilizing software and artificial intelligence. When the FDA first introduced the term “Predetermined Change Control Plan” in its April 2019 discussion paper1 titled Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) - Discussion Paper and Request for Feedback, the initial goal was to develop a new regulatory framework to unlock the huge real-time continuous improvement potential of AI/ML technologies using the vast amount of real-world data generated during the delivery of healthcare after an AI/ML-enabled device is released to the market. The same framework can help the FDA and the medical device industry to accelerate the pace of innovation with many other devices while maintaining device safety and effectiveness.
In fact, when Congress provided the FDA with formal legal authorization to manage postmarket device changes using PCCP through the passage of the Food and Drug Omnibus Reform Act of 20222, the scope of PCCP extended far beyond AI/ML-enabled software. The FDA was authorized to leverage PCCP to manage any and all types of postmarket changes that otherwise require premarket submissions for such changes, as long as the intended use of the device is conserved, safety and effectiveness of the device are not adversely affected, and for the 510(k) devices, substantial equivalence is preserved as well.
An authorized PCCP allows the FDA to pre-clear or pre-approve predefined specific changes (“modifications”) to previously cleared or approved devices, without the need for a new premarket submission for each change, when the manufacturer follows the predetermined procedures (“modification protocols”) to develop, verify, validate, and implement the changes.
The FDA published a draft guidance3 on April 3, 2023, titled Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence/Machine Learning (AI/ML)-Enabled Device Software Functions (also known as AI/ML PCCP draft guidance). As the guidance title suggests, this first PCCP guidance (in draft form) only applies to AI/ML-enabled software functions.
Based on the research Bridging Consulting recently conducted, as of June 30, 2024, the FDA has authorized a total of 38 PCCPs spanning various device types (including but far beyond AI/ML-enabled software) and submission categories. This article will delve into the regulatory search results for published 510(k) summaries, de novo classification summaries, and PMA approval orders, presenting a detailed statistical analysis of these PCCPs.
Regulatory Search Methodology
To understand the impact and scope of the PCCP, we conducted comprehensive regulatory research. The following FDA databases were used for this regulatory research project:
We also used Basil Systems,4 a web-based SaaS platform that contains all FDA data, leveraging an artificial intelligence-powered engine to index, correlate, and cross-reference regulatory, quality, and clinical data. In addition, a general Google search was conducted to search for company and/or FDA announcements and other research articles and analyses regarding PCCP. It is worth noting that the search results below may not be exhaustive as we observed wide variations in how and whether “Predetermined Change Control Plan” or “PCCP” were referenced and/or described in premarket submission decision summaries, such as 510(k) summaries.
Search Results
Our search identified a total of 38 authorized PCCPs to date, providing insights into the distribution and characteristics of these plans. The statistical breakdown is as follows.
1. Classification Panels
The authorized PCCPs cover a diverse range of device classification panels, reflecting the wide applicability of the PCCP framework across different medical specialties. The breakdown is as follows:
Radiology: 9
Cardiovascular: 7
Orthopedic: 5
Microbiology: 3
General and Plastic Surgery: 3
Pathology: 3
Neurology: 2
Obstetrical and Gynecological: 2
Chemistry: 1
Dental: 1
Gastroenterology and Urology: 1
Ear, Nose and Throat: 1

2. FDA Review Divisions
All eight Office of Health Technology (OHT), also referred to as “review divisions” under CDRH’s Office of Product Evaluation and Quality (OPEQ), have authorized numbers of PCCPs with varying experience. The breakdown is as follows:
OHT1: 2
OHT2: 7
OHT3: 3
OHT4: 4
OHT5: 2
OHT6: 5
OHT7: 7
OHT8: 9

3. Premarket Submission Types
The PCCPs have been authorized through various premarket submission types, including 510(k), de novo, and PMA submissions. The distribution is:
510(k): 32
De Novo: 2
PMA: 4

4. Device Types
The types of devices covered by PCCPs include hardware, software, systems (i.e., devices including hardware and firmware/software), and in vitro diagnostics (IVD). The analysis shows:
Hardware: 15
Software: 12
System: 5
IVD: 6

5. AI/ML-Enabled Software
The authorized PCCPs started with AI/ML-enabled software as a medical device (SaMD) and quickly covered many other devices without AI/ML-enabled functions. The breakdown is as follows:
AI/ML-Enabled SaMD: 10
Device without AI/ML-Enabled Function: 28

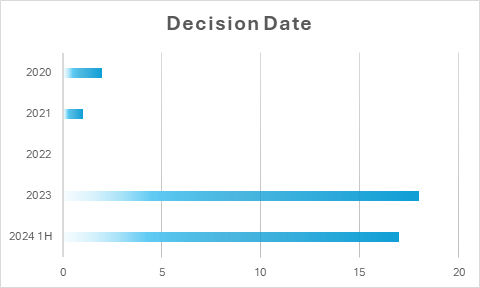
6. Decision Dates
The timeline of PCCP decisions shows the evolution of the framework over the past five years. The decisions are broken down by year:
2020: 2
2021: 1
2022: 0
2023: 18
2024 (January through June): 17
7. PCCP Details in Decision Summaries
The level of detail provided in PCCP decision summaries varies, with some offering comprehensive insights while others are more limited. The categories include:
No PCCP Information: 23
Limited PCCP Information: 10
Detailed PCCP Description: 5

General Trends
The regulatory search results and the statistical data reveal several trends in the PCCP landscape. For example:
PCCP did not suddenly appear under the sun. The FDA and various device companies had worked out plans, protocols, and, in some cases, exemptions for managing postmarket device changes. DEN1900405 is a good example.
PCCP submissions and authorizations have picked up speed in the last 12 months. The FDA authorized a similar number of PCCPs in the first half of 2024 as in the entirety of 2023.
Even though the FDA only published one draft PCCP guidance on AI/ML-enabled software, companies were not waiting, and the agency has been quite accommodating in leveraging this legally available tool to reduce postmarket regulatory submission burden for both companies and the FDA.
Although the AI/ML PCCP draft guidance3 states “the PCCP should be described in the 510(k) summary, Do Novo decision summary, or PMA summary of safety and effectiveness document (SSED) and approval order,” a requirement that is critical to promote transparency, it has not been followed consistently across all CDRH review divisions. Our search only identified five 510(k) summaries6 with sufficient details, four of which are for AI/ML-enabled SaMDs.
Some of these patterns highlight that we are still in the early days of leveraging this new regulatory tool for both the industry and the FDA.
Other Notable PCCP Developments
Our research also identified several other notable developments:
1. PCCP Incorporated into Class II Special Controls
As established through DEN1900405 classification request for Caption Health Inc.’s Caption Guidance, one of the special controls for “radiological acquisition and/or optimization guidance system” devices regulated under 21 CFR 892.2100 is for a PCCP:
Design verification and validation must include:
d. A detailed protocol that describes, in the event of a future change, the level of change in the device technical specifications or indications for use at which the change or changes could significantly affect the safety or effectiveness of the device and the risks posed by these changes. The assessment metrics, acceptance criteria, and analytical methods used for the performance testing of changes that are within the scope of the protocol must be included.PCCP is an optional tool that can be used by device companies to receive preclearance or pre-approval from the FDA for specific future device changes. Although special control 1.d for 21 CFR 892.2100 does not specifically use the term “PCCP,” the “detailed protocol” required seems to many to be a PCCP. While the FDA does not have the authority to require the use of an optional regulatory tool, by including this in the required special controls for this whole new classification of the AI/ML-enabled guidance systems, the FDA established the expectation of requiring greater rigor in ensuring that appropriate device development process, risk assessment, and performance criteria information are included in 510(k) submissions to effectively manage premarket clearance for similar devices. This is an interesting use of the PCCP.
It is worth noting that the decision date for DEN190040 was Feb. 7, 2020, nearly two years prior to formal Congressional PCCP authorization for the FDA.
2. PCCP Incorporated into Device-Specific Guidance Document
The FDA issued a guidance on September 29, 2023, titled Antimicrobial Susceptibility Test (AST) System Devices – Updating Breakpoints in Device Labeling, replacing an earlier version from June 2009. This guidance describes approaches for AST system device sponsors to update their susceptibility test interpretive criteria (STIC) in device labeling via PCCPs.
Section 515C of the FD&C Act provides that the FDA may require that a PCCP include labeling for safe and effective use of a device as such device changes pursuant to such plan, notification requirements if the device does not function as intended pursuant to such plan, and performance requirements for changes made under the plan.
This guidance requires that all breakpoint change protocols submitted and cleared in future 510(k) submissions be authorized in the form of PCCPs. The impact from this new guidance is still unknown, as the FDA had reviewed and cleared many 510(k)s for new AST system devices with “breakpoint change protocols” prior to this guidance. Renaming such protocols as PCCPs may increase the rigor of documentation requirements.
3. PCCP Guiding Principles
In October 2023, the U.S. FDA, Health Canada, and the U.K. Medicines and Healthcare products Regulatory Agency (MHRA) jointly identified five guiding principles8 outlining key characteristics of robust PCCPs:
Focused and bounded
Risk-based
Evidence-based
Transparent
Total produce life cycle (TPLC) perspective
Predictions On Future Trends
Looking ahead, the PCCP framework is likely to expand and evolve. We anticipate several developments:
Broader PCCP adoption across various medical device categories.
Device companies will likely leverage PCCPs for all types of postmarket changes, including design changes, shelf-life extension, adding compatibility and interoperability, manufacturing process or facility changes, or even expanding indications for use using real-world data in certain cases, all while preserving the intended use. The utility of the PCCP is almost limitless.
The FDA will finalize the AI/ML PCCP draft guidance and likely issue new guidance for other device types, trying to drive consistency related to requirements including the public disclosure requirement. FDA may also update existing 510(k) and PMA modification guidance documents to incorporate PCCP into the postmarket change submission decision-making process.
After overcoming the initial learning curve, companies will utilize PCCP only in select cases where there is a positive return on investment (ROI), as a successful PCCP requires a significant amount of initial planning, time, and resource investment.
These trends suggest that the PCCP will continue to play a crucial role in medical device regulation, fostering innovation while ensuring patient safety and product effectiveness.
Conclusion
The PCCP marks a pivotal shift in medical device regulation, offering a proactive approach to managing changes to devices. It is part of a broader effort to carefully balance innovation with regulatory oversight in a deliberate and controlled manner.
By examining the current status and analyzing the trends, we gain valuable insights into the future direction of this initiative. As the PCCP framework continues to evolve, it will be essential for manufacturers, regulators, and stakeholders to collaborate closely to maximize its potential benefits.
References
Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) - Discussion Paper and Request for Feedback. U.S. Food & Drug Administration; 2019.
Consolidated Appropriations Act 2023. Vol SEC. 3308. PREDETERMINED CHANGE CONTROL PLANS FOR DEVICES.; 2022.
Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence/Machine Learning (AI/ML)-Enabled Device Software Functions - Draft Guidance for Industry and Food and Drug Administration Staff, issued on April 3, 2023. U.S. Food & Drug Administration; 2023.
DEN190040, Caption Guidance. Caption Health, Inc.; 2020.
(a) K231173, IRNF. Apple Inc. 2023; (b) K232603, CamAPS FX. CamDiab Ltd. 2024; (c) K233030, BoneMRI. MRIGuidance B.V. 2024; (d) K233216, CLEWICU System. Clew Medical Ltd., 2024; (e) K233438, SleepStageML. Beacon Biosignals, Inc. 2024.
Antimicrobial Susceptibility Test (AST) System Devices – Updating Breakpoints in Device Labeling – Guidance for Industry and Food and Drug Administration Staff, issued on September 29, 2023. U.S. Food & Drug Administration; 2023.
Predetermined Change Control Plans for Machine Learning-Enabled Medical Devices: Guiding Principles. U.S. FDA, Health Canada, U.K. MHRA; 2023.



Comments